Dive Brief:
- U.S. pharmaceutical company Micron Biomedical secured a $23.6 million grant from the Bill & Melinda Gates Foundation to fund mass production of needle-free vaccines that administer medicine via a patch style delivery.
- The funds will allow Micron to increase its manufacturing capacity and commercialize needle-free vaccine delivery, according to a company press release.
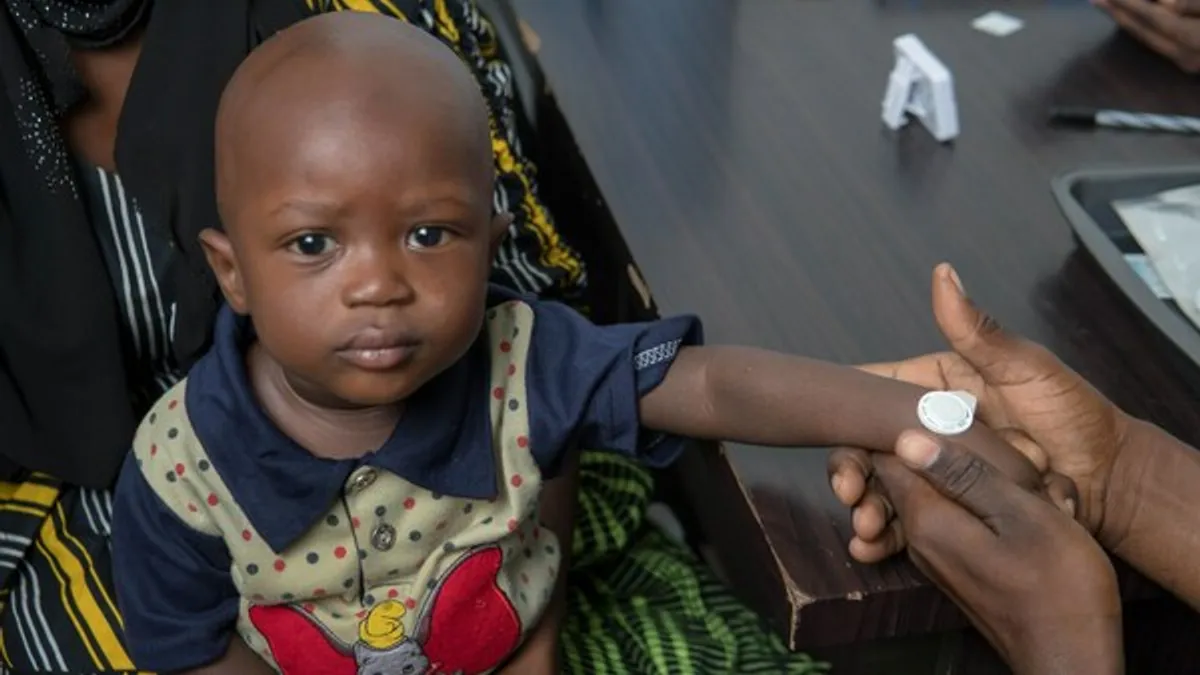
- The innovative technology intends to expand the accessibility of a measles-rubella vaccine in developing countries, where the disease is most common.
Dive Insight:
This funding builds upon a previous 2017 grant from the Bill and Melinda Gates Foundation that supported the first-ever clinical trial of Micron's microarray technology in children earlier this year.
The successful clinical trial in Gambia showed that Micron’s device delivered the measles-rubella vaccine to adults and infants as young as nine months old as safely and effectively as syringes, according to the company. The pharmaceutical firm also says the method produced a similar immune response.
Micron claims the needle-free method is easier to transport and administer, because it does not require refrigeration, unlike needle vaccines. The new technology “reduces the need for a cold chain and allows a community health worker to vaccinate a child within minutes by applying the technology to the skin and pressing a button that confirms administration,” the company said in its announcement.
While the company has completed Phase 1 and 2 of the trial, it’s still waiting on approval for the third and final phase of commercial-scale manufacturing. Micron gave no specific timeline on when the World Health Organization and other “appropriate regulatory authorities” will approve the vaccine, but said it’s working “as quickly as possible,” Micron CEO Steve Damon said in an email statement.
The biomedical company will develop equipment for at least 10 million patch-like devices a year and will expand output if necessary. Micron’s clinical manufacturing is in Atlanta, Georgia, while its medical equipment production is in Germany. The company plans on initially hiring contracted manufacturing organizations for the large-scale project.
“We haven't finalized all our CMO’s or locations yet. We’re building our equipment in Germany and in the US, which will then be placed with our CMO(s) for the initial Measles & Rubella product as well as the other products in our pipeline,” Damon stated in the email.
The initiative addresses the challenge of limited vaccine access in low- and middle-income countries where children have the highest risk of death from measles, according to the Centers for Disease Control and Prevention.
"By supporting Micron's efforts to develop a state-of-the-art, high-quality and large-scale production facility to manufacture our microarray technology, we have an opportunity to greatly improve on access and availability of measles/rubella vaccines, as well as other traditionally-injectable Global Health vaccines, in underserved populations around the world,” Steven Damon, CEO of Micron Biomedical, said in a statement.